Tuesday, 30 January 2018
Applications (Uses) of Zeolites in Civil Construction and other Fields
January 30, 2018Applications(Uses) of Zeolite, Learning Material, Mine Waste Management, Zeolite Ore
2 comments

Besides the widely usage of zeolites in the
Livestock and Agriculture Industries, it is also utilized in the civil constructions
and other technological fields as well.
To begin with, zeolites can also be used for
separating oxygen and nitrogen in the air to produce oxygen-enriched gas. This
technology utilizes the strong absorbability of mordenite to nitrogen molecules
to generate oxygen continuously which is vastly depend on the device and can
also produce nitrogen as well.
Zeolite rock/ore is generally soft but not
fragile is used as teaching material for carving for school children and is
also used as cleanser.
One of the new applications of zeolites is in
food packaging materials that are made of polyethylene film incorporated with zeolites
was developed with an aim to retain the freshness of food or fruits and vegetables.
One of the most important applications of zeolites
is in the civil constructions. In civil constructions, zeolites are used as new
lightweight building materials such as plastic filler and plywood adhesives.
Further to the lightweight, zeolites are also used as foam blocks incorporated
in linings of chimneys of thermal plants and dolomite plaster.
Similar to zeolite, siliceous mud-stone is
utilized in such applications as soil conditioners, deodorizers (for
environment improvement of poultry and pig farms), and special fertilizers.
Siliceous mudstone is also referred to as “crystobal
rock” which contains a lot of crystobalite composed of silica (SiO2).
Zeolite is used in a wide range of applications
and among them is the clay for papermaking which was developed in Japan.
Zeolite is soft and its powder is significantly white and it is applied to
papermaking to take advantage of its properties. Zeolite clay is serving as a
filter which provided paper with high opacity and excellent ink acceptance.
Synthetic zeolite was invented by German
Professor Gans in 1905, and was first named permutit and after permutare, a
Latin word meaning exchange. Permutit has a composition of Na2O.Al2O.xSiO2.yH2O
and strong adsorbability.
References:
Boiling stone (zeolite), Zeolite
Dynamics, Lecture Notes.
Nouko to Engei (Agriculture and
Horticulture), September 1978.
JACK News
Materials for Zeolite Forum (1989)
Survey by Zeolite Industrial
Association
Applications (Uses) of Zeolites in the Livestock and Agriculture Industries.
January 30, 2018Applications(Uses) of Zeolite, Learning Material, Mine Waste Management, Mine Waste Treatment, Zeolite Ore
No comments

Zeolites are widely used in the livestock and agriculture
industries among other uses. In this
article, it discusses the common uses of zeolites in the livestock and
agriculture industries. In the Livestock
Industry, zeolite is purposely used for improving the growth and health of animals
while in the Agriculture Industry, zeolite is used for the purpose of improving
the fertility of the soil for healthy growth of cash crops.
The use of zeolites in the livestock industry is
basically added to the livestock feed which absorb the toxins in the body of
chickens and pigs and finally discharge them out of the body.
Furthermore, the minerals contained in the
zeolites actually promote the growth and health of livestock. That is, zeolites
adsorb the stench of ammonia to improve the environment and also function as
drying aids by virtue of their ability to absorb moisture. Thus, cat litter (litter
for pets) utilize such properties of zeolites for optimum benefits.
In the Agriculture Industry, it utilizes the
properties of zeolites such as the adsorption capability and Base Exchange capability
which prevent the outflow of fertilizers by absorbing the components of fertilizers
as well as improving soils by neutralizing soils containing acids or acidic
soils. Zeolites also have excellent absorption ability and water retention capacity
which make them more effective in preventing either drought or cold weather
impacts of any kind cause by nature.
Estimation of cation exchange capacity of
zeolite is much more difficult as it is closely related with qualities from its
appearance. As such, zeolite powder is not readily distinguished from other
rock powder.
Zeolite is specified as land improvement
material by a Cabinet Order based on the Soil fertility Enhancement Act (Act No.34
of 1984) in order to assure the quality. According to Cabinet Order, the
zeolite must meet the criterion “cation exchange capacity (C.E.C.) per 100 g of
dry matter is 50g or more.”
Moreover, the applications or uses of zeolites
range from water purification for fish farming to pollution prevention
exercises. The water purification for fish farming refers to a function to
adsorb ammonia and hydrogen sulfide in water which produces purified water. In addition to that, zeolites have an effect
of softening hard water to stabilize pH.
Besides water purification for fish farming,
zeolites are also used for pollution prevention in the following fields:
·
Purification of hazardous components
in industrial wastewater.
·
Adsorption treatment of heavy metal
ions from abandoned mines.
·
Removal of heavy metals from plating
waste.
·
Decolourization of waste water from dye
houses.
·
Prevention of eutrophication of
lakes and marshes caused by ammonia nitrogen.
·
Removal of harmful components from
automobile exhaust gas.
The supply of zeolites depends entirely on
clients' demand and off course consumption rate. Supply of zeolites is also
dictated by scale of mining operation and processing.
 |
| Zeolite ore (rock) |
References:
Boiling stone (zeolite), Zeolite
Dynamics, Lecture Notes.
Nouko to Engei (Agriculture and
Horticulture), September 1978.
JACK News
Materials for Zeolite Forum (1989)
Survey by Zeolite Industrial
Association
Sunday, 28 January 2018
Data Analysis of Rapid PACKTEST Results
January 28, 2018Learning Material, Mine Pollution, Mine Waste Management, Rapid PACKTEST, Water Quality Monitoring Technique
No comments

Upon the completion of the setup and PACKTEST, you have the data
available at hand to record. In your note book you record the readings of pH,
Turbidity, Temperature, metal conductivity. Then you have the other sets of
data from the PACKTEST results. Each element tested has got a numbered color
range that corresponds to the concentration of that element/compound in the
water sample. You record every data for the elements that are tested.
 |
| Table 1 Rapid PACKTEST Results |
Be careful to graph the related data so that any conclusion drawn can
make sense with respect to particular information. i.e. if you plot all the
data into one graph then always take note of the legends so that your
interpretation is accurate in reporting.
It is also better to take readings at different weather patterns
i.e. during rainy seasons and dry seasons. Remember to keep the sample points
unchanged so that a good comparison is made.
From the data analysis, it is better to make few comments and off
course a recommendation is anticipated from the field investigation. Your
recommendation should provide a clear direction/ indication should there be precautions
taken within the vicinity of the impacted project area especially the mine
impacted communities. Your recommendation should also alert the local
government authorities regarding the findings and what to do in that part of the
area.
Characteristics of Zeolite Ore
January 28, 2018Learning Material, Mine Pollution, Mine Waste Management, Mine Waste Treatment, Zeolite Ore
No comments

- Like any other matters, Zeolite has two major characteristics which are:
1. a chemical composition having
zeolitic water, and
2. An excellent ion exchange
capability.
Zeolitic water is unique in the composition
which is hardly observed in other minerals, as dehydration occurs without
changing the crystal structure under heating. This hydration behaviour enables
zeolite to be used as moisture absorbent.
In addition, the dehydrated
zeolite has a myriad of holes like a honeycomp and the holes have such small
sizes in the order of angstroms (Symbole:Å,
unit cm/100 million).
Accordingly a mixture of gasses with different molecular sizes, which are
chemically difficult to separate at the molecular level, can be sieved through
zeolite. This is called the “molecular sieve effect.”
The Cation exchange
capacity of zeolite is explained as follows:
The general chemical
composition of zeolite in general is indicated by
(M2+,M2+)O.Al2O3.mSiO2.nH2O.
The symbols
in the parentheses at the beginning of the formula indicate exchangeable
cations. Cations in zeolite are exchangeable with other cations in aqueous
solutions. In chemical terms, material with a positive charge is referred to as
a base. The ability to exchange bases is Base Exchange capability (or cation
exchange capability), while the capacity to exchange bases is base exchange
capacity (or cation exchange capacity) and is called C.E.C., the acronym for
“Cation Exchange Capacity.”
Ion exchangeability allows
silicon (Si) atoms located in the centre of the zeolite crystal lattice to be
partially replaced with aluminium (Al) atoms, resulting in the loss of cations.
Cations such as sodium (Na), Calcium (Ca) are captured in the crystal lattice
to compensate for the shortage.
The function of C.E.C. is
similar to that of the liver of an animal which stores nutrients. Zeolite
adsorbs and stores fertilizer components (bases) such as sodium, potassium and
calcium to supply the nutrients (fertilizer components) to crops in response to
request.
The unit of cation exchange
capacity is represented by mg equivalent (meq) per 100g of soils or zeolite
rocks.
The cation capacities of clay
minerals are different depending on the type of minerals. Montmorillonite is
the main mineral source for bentonite which exhibits the highest C.E.C. after
zeolite.
Reference:
Boiling
stone (zeolite), Zeolite Dynamics, Lecture Notes.
Roskill
Report (1990)
Annual
Reports of Various Companies.
Nouko
to Engei (Agriculture and Horticulture), September 1978.
JACT
News.
Friday, 26 January 2018
Localities of Zeolite Ore
Since all Zeolites are alteration products of volcanic glass present in tuff (a rock containing consolidated volcanic ash) or tuff breccia (rock consists of volcanic rocks cemented together by large amount of volcanic ash), the ore does not consist solely of zeolite, and thus it is appropriate to be called zeolite-containing tuff.
Zeolite resources can be found in sedimentary layers or rocks of volcanic ash throughout the world. Natural zeolite deposits have recently been discovered in the Pacific Rim countries, including New Zealand and countries of Mediterranean coast. Among these countries, Japan has been leading the rest of the world in the exploration and development of natural zeolite and has been the world’s major producing countries.
Localities of Natural zeolite in the United States are distributed mainly in the western states such as Oregon, Nevada, California and Idaho. Most of the mines produce clinoptilolite and mordenite
Apart from United States and Japan, zeolite is also discovered in Eastern European countries which include eastern part of Czechoslovakia, the north-eastern part of Hungary, the north-western part of Yugoslavia, and south-eastern part of Bulgaria. And also countries like Italy, Cuba, Brazil, South Africa and China are all recorded as zeolite producing countries.
Australia recently started producing natural zeolite in Werris Creek, South of Tamworth in New South Wales as well as Cranky Corner near Singleton.
Zeolite is purified differently depending on the nature of the ores and is associated to the processes of granulation, drying, milling, screening (sizing) and bagging. Special applications such as filler for high-quality paper, after milling, the zeolite is subject to wet process of bleaching, concentration, filtering and finally drying.
Reference:
Boiling stone (zeolite), Zeolite Dynamics, Lecture Notes.
Roskill Report (1990)
Annual Reports of Various Companies
Thursday, 25 January 2018
Zeolite (Boiling Stone) Ore
January 25, 2018Learning Material, Mine Waste Management, Mine Waste Treatment, Zeolite Ore
No comments

Zeoilte (Boiling Stone) Ore
Zeolite is a term referred to as Boiling Stone.
The Term Zeolite is derived from two Greek words Zeo (to boil) and Lite (a
stone). The heated ore supported at the
tip of a blowpipe which is used for qualitative research is observed to swell
into a pumice-like porous state when air is blown from the mouthpiece. The
phrase boiling term comes from this behaviour.
Zeolite is an aluminosilicate mineral (silicate
in which silicone atoms are partially replaced with aluminium atoms) containing
maily alkali metals such as sodium and potassium and alkaline earth metals such
as calcium and magnesium, as well as water molucules (H2O) in the
form of crystals.
(M2+,M2+)O.Al2O3.mSiO2.nH2O
M2+: Mainly Calcium (Ca)
M+: Mainly Sodium (Na),
Potassium (K)
Zeolite commonly occur in pores of volcanic
rocks or inside the rocks in the shape of veins, and are found in the strata
near metal ore deposits as well as geothermal power plants and hot springs.
Volcanic glass in tuff often transforms into zeolite under the influence of
seawater. As a result, besides pure zeolite components, zeolite rock contains
minerals such as clay (montmorillonite), iron oxide and feldspar.
Generally, zeolite will generate different
zeolite crystal structures, even if it is from the same origin rock, depending
on the burial depths, with the pressure on the rock increases to affect the
crystal structure with and aid of ground water and hot water. Natural
Among many minerals, the widely distributed
natural zeolites are clinoptilolite (often called ‘clino’) and mordenite.
Laumontite is a white plate-like or columnar
crystal. Laumontite, which is formed primarily by the action of hot spring
water, replaces minerals in rocks or fill cracks making a pattern of veins.
Laumontite can be found in aggregates (or gravel) in concrete.
Laumontite is said to react with alkali in
cement (alkali-aggregate reaction) to inflate the aggregate which cause
cracking.
Applications of zeolite will be in the next article.
Applications of zeolite will be in the next article.
Reference:
Boiling stone (zeolite), Zeolite Dynamics, Lecture Notes.
 |
| Zeolite ore |
Tuesday, 16 January 2018
How to Conduct Rapid PACKTEST in a Well (below 1 meter in depth)
January 16, 2018Learning Material, Mine Waste Management, Mine Waste Treatment, Rapid PACKTEST, Water Quality Monitoring Technique
No comments

Well is simply a shaft sunk into the ground or built upwards from
certain depths below a natural surface of the ground which extends further
above certain heights of the natural surface of the ground
Wells can be naturally
occurring or man-made. Man-made wells are created to suite the desired
purpose(s) of the organisation or individuals. Wells can be created to collect water
or oil or gas below the earth’s surface.
In a mining operation, wells are created for the purposes of heap
leaching through a vat. Heap Leaching is one of the various mining techniques
to extract gold from the ore of various host rocks using cyanide which is one
of the common solvent in this gold recovery technique.
During the gold recovery process, cyanide is sprinkled over the
heap of crushed gold bearing gravels (ore) to dissolve the gold into a pregnant
solution or into liquid form. The pregnant solution is then sucked out through
the vat and further into carbon columns and take to the processing plant for
further processing and smelting.
Upon the closure of the mine, the vats are no longer in use. Cyanide
is left behind the pool beside the heap-vats eventually gets into the surrounding
environment which is a concern for mine waste management. Water is then filled
through the Vats up to certain heights. So
from the top and surface of the heap-vats, the water level is below certain depths
of about 2 – 3 meters which are hardly reached by hand. And also the diameter
of the vats is about 30-40 cm which is too narrow to be accessed. So how can
you how can you overcome this challenge to take a water sample for a Rapid PACKTEST
as well as other measurements?
The simple way to get sample is by utilizing the following
equipment procedures:
1. String line (rope)
2. Metal weight(1kg weight)
3. 3x1 Litre plastic bottle(container)
4. Masking tape
5. Water level measuring tape
6. GPS
7. Note book, pen, pencil
8. Camera
9. Blade/kitchen knife
11. Syringe
12. 0.45µm
filter
13. turbidity
meter
14. pH meter
15. Laptop/computer
Procedure
1. Cut the 1 Litre plastic bottles (container)
into more than half.
2. Tie the 1kg metal weight at the tip of the
string line.
3. With the masking tape, fasten the cut container
with the weight attached to the string line.
4. Drop the container attached to the weight
and stringline into the well and allow the container to be filled with water.
5. Pull the string line with all its attached
items and pour the fetched water into the other reserved containers.
6. Never forget to measure the water level by
using the 50m water level measuring tape.
7. Using the GPS you take the readings of
sample location coordinates and altitude and location zone.
8. Finally , you conduct Rapid PACKTEST and
9. Turbidity and pH measurements,
conductivity and temperature readings as well.
The above procedure can be repeated for wells or pools that are
hardly accessible in person or by hand.
Data Collection of the Rapid PACKTEST is the final thing to do before moving to the
next location or ending the field work.
Data Collection of Rapid PACKTEST Results
January 16, 2018Learning Material, Mine Pollution, Mine Waste Management, Rapid PACKTEST, Water Quality Monitoring Technique
2 comments

Data collection is the recording and assembling of results obtained from the Rapid PACKTEST experiments at various locations.
Before you conduct the packtests, you need to have the required materials for preparation, experiment and recording of data. The materials and equipment you would require for testing include but not limited to:
Packtest Kits.
GPS
Note Book
Pencil/pen
3 x Half cut container
syringe
0.45µm filter
Camera/smart phone with camera.
turbidity meter
pH meter
Laptop/computer
Once you have the above equipment list, you are about to conduct the PACKTEST and other necessary measurements. But before that you must never forget to give a sample location name or sample code/ID and record the coordinates, location zone and offcourse altitude of the sample location. This will ease the management of various data of the same type.
Thereafter, you proceed with the PACKTEST procedures as outline below which is a global practice:
Sampling Procedure for PACKTEST
1. Fetch water in container and filter the water using syringe and 0.45µm filter to filter water sample and pour filtered water sample into a clear mini cylinder(half cut container).
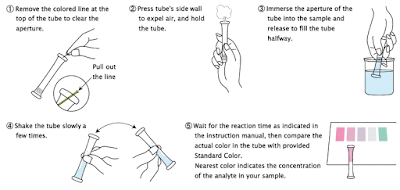
2. Remove the colored line at the top of the tube to clear the aperture.
3. Press tube's sidewall to expel air, and hold the tube.
4. Immerse the aperture of the tube into the water sample in the mini cylinder and release to fill the tube halfway.
5. Shake the tube slowly for few seconds.
6. Wait for the reaction time as indicated in the instruction manual, and then compare the actual color in the tube with provided Standard Color. The nearest color indicates the concentration value (mg/L = ppm) of the analyte in your sample.
Concurrently set up the equipment for measuring the pH, temperature, metal conductivity and turbidity of the water sample at the same sample location for the packtest water sample taken. After the setup is complete, you take the reading carefully as the reading varies every second. It is recommended to take the best average reading.
Upon the completion of the setup and PACKTESTs, you have the data available at hand to record. In your note book you record the readings of pH, Turbidity, Temperature, metal conductivity. Then you have the other sets of data from the PACKTEST results. Each element tested has got a numbered colour range that corresponds to the concentration of that element/compound in the water sample. You record every data for the elements that are tested.
Finally you need to digitise the data and analyse the test results. To do that, you need to have a computer or a laptop. Create an excel spread sheet in your laptop or computer and enter the field data in a tabulated format. Your sample results look like the table below:
How to do data analysis from the PACKTEST results and other measurements is in a different article (Data Analysis of Rapid PACKTEST Results)
Related Articles:
Rapid PACKTEST
Data Collection of Rapid PACKTEST Results
Data Analysis of Rapid PACKTEST Results
How to Conduct Rapid PACKTEST in a Well
Before you conduct the packtests, you need to have the required materials for preparation, experiment and recording of data. The materials and equipment you would require for testing include but not limited to:
Packtest Kits.
GPS
Note Book
Pencil/pen
3 x Half cut container
syringe
0.45µm filter
Camera/smart phone with camera.
turbidity meter
pH meter
Laptop/computer
Once you have the above equipment list, you are about to conduct the PACKTEST and other necessary measurements. But before that you must never forget to give a sample location name or sample code/ID and record the coordinates, location zone and offcourse altitude of the sample location. This will ease the management of various data of the same type.
Thereafter, you proceed with the PACKTEST procedures as outline below which is a global practice:
Sampling Procedure for PACKTEST
1. Fetch water in container and filter the water using syringe and 0.45µm filter to filter water sample and pour filtered water sample into a clear mini cylinder(half cut container).
2. Remove the colored line at the top of the tube to clear the aperture.
3. Press tube's sidewall to expel air, and hold the tube.
4. Immerse the aperture of the tube into the water sample in the mini cylinder and release to fill the tube halfway.
5. Shake the tube slowly for few seconds.
6. Wait for the reaction time as indicated in the instruction manual, and then compare the actual color in the tube with provided Standard Color. The nearest color indicates the concentration value (mg/L = ppm) of the analyte in your sample.
Concurrently set up the equipment for measuring the pH, temperature, metal conductivity and turbidity of the water sample at the same sample location for the packtest water sample taken. After the setup is complete, you take the reading carefully as the reading varies every second. It is recommended to take the best average reading.
Upon the completion of the setup and PACKTESTs, you have the data available at hand to record. In your note book you record the readings of pH, Turbidity, Temperature, metal conductivity. Then you have the other sets of data from the PACKTEST results. Each element tested has got a numbered colour range that corresponds to the concentration of that element/compound in the water sample. You record every data for the elements that are tested.
Finally you need to digitise the data and analyse the test results. To do that, you need to have a computer or a laptop. Create an excel spread sheet in your laptop or computer and enter the field data in a tabulated format. Your sample results look like the table below:
 |
| Table 1: Rapid PACKTEST Results |
Related Articles:
Rapid PACKTEST
Data Collection of Rapid PACKTEST Results
Data Analysis of Rapid PACKTEST Results
How to Conduct Rapid PACKTEST in a Well
Thursday, 28 December 2017
Ramu Nickel Mine Prepares for Royalty Payment
The Ramu Nickel and Cobalt Mine in Kurumbukari, in Madang Province is preparing to pay long waited royalty to the landholders. This was reported in the National News Paper dated 28th December 2017.
Landholder Royalty for Ramu Nickel Project has not been paid since shipment till to date. This delay could be due yo the tax holiday given to the developer by the government or as a condition in the Mining Developments Contract(MDC).
This matter was brought to light but it was further delayed since then to settle administrative processes. It is now looking promising as the developer takes initiative to organize the landholders in preparation for royalty and other benefits that are ongoing.
The Mining Act 1992 is somewhat drafted in way as to cater for landholders grievances and expectations. Like in the case of landowner royalty, it will he paid directly into their accounts rather than landowners front the company's office or government's office to get their rightfully due payments.
Credits to MCC for this step towards royalty payment.
Sunday, 24 December 2017
Rapid PACKTEST
December 24, 2017Learning Material, Mine Waste Management, Rapid PACKTEST, Training, Water Quality Monitoring Technique
No comments

Rapid PACKTEST is commonly known as PACKTEST. This test is a simple onsite method of testing water quality which produces amazing results in a split of a second. It does not require complicated analytical techniques to determine the quality of water. Concentration of heavy metals, precious metals and any other elements and compounds in the water are detected using this method. pH of water can also be determined using this method. Water in this case can be a flowing creek,settling ponds, pool beside vats or heap leach pads, mine pits etc.
 |
PACKTEST Results for Pihema Creek,
Morobe Province. Cyanide Detected
(Dark-blue).
|
It is recommended
that Pact Test should be practiced by all government regulators in the
extractive industries especially in the Mining and petroleum industry. This
will greatly help during statutory inspections which can give results instantly
onsite rather than waiting for results in the laboratory after few weeks or
months. This will also be a alert for the industry to treat waste water
effectively or improve on their waste water treatments and monitoring
techniques.
The process and the
setup of the testing method are outlined below.
Sampling Procedure for PACKTEST
1.
Fetch
water in container and filter the water using syringe and 0.45µm filter
to filter water sample and pour filtered water sample into a clear mini
cylinder.
2.
Remove the colored line at the top of the tube
to clear the aperture.
3.
Press
tube's sidewall to expel air, and hold the tube.
4.
Immerse
the aperture of the tube into the water sample in the mini cylinder and release
to fill the tube halfway.
5.
Shake
the tube slowly for few seconds.
6.
Wait
for the reaction time as indicated in the instruction manual, and then compare
the actual color in the tube with provided Standard Color. The nearest color
indicates the concentration value (mg/L = ppm) of the analyte in your sample.
Note: Cyanide is tested differently. Before the above procedure is applied,
first of all dissolve the mixing reagent into the filtered water in a small enclosed
translucent cube of about 2cm x 2cm x 1.5cm in size. Finally immerse the
cyanide reagent into the solution and release to fill up the tube. Shake the
tube and record the reading after 8 minutes.
The reaction times for
each element or compound varies from seconds to minutes. Reaction time for
cyanide takes longer than any other elements or compounds. It could be other
elements or compounds as well but during the tests conducted by the reporter
indicated that cyanide took longer than other elements.
It is recommended that
PACKTEST should be conducted on site. It would be a bad practice if sample is
taken from a different spot and date and tested on different date and location
as the results would not represent the sample location and time.
If you do then be aware to note the results and anticipate error in the
readings recorded.
Other Equipment for
testing water quality includes but not limited to pH meter, Turbidity Meter. pH
meter is for measuring the pH level of water
and also measure the temperature of water. Turbidity meter measures
turbidity of water and also temperature and metal conductivity in the water.